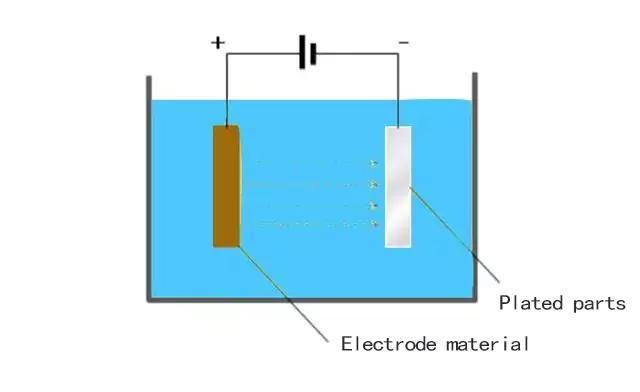
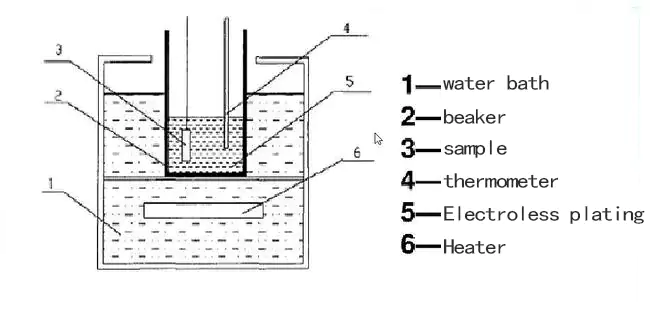
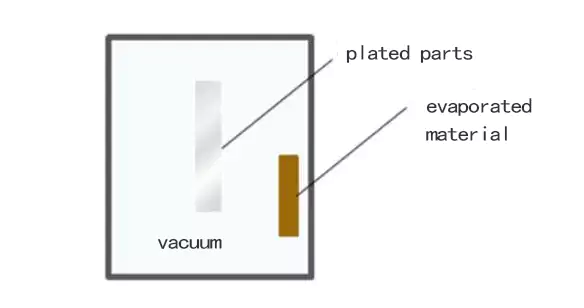
How Electroplating Works and Where it is Used

Table of Contents What is Electroplating? How Does Electroplating Work? Electrolysis Electrodes Plating bath Deposition of the metal Types of Electroplating Metals for Electroplating Electroplating and Tolerances Edge build-up Barrel Plating vs Rack Plating Hard-to-flow areas Electroplate Plastics How are…