Aluminum Anodizing Color: Anodizing Principle, Type, Colors, and More
Anodizing is a process that uses an electrochemical reaction to form an oxide film on an anode metal. The anodizing process improves the durability, corrosion resistance, and aesthetics of aluminum products. This article will focus on the aluminum alloy’s anodizing and discuss the various types of anodizing, how to obtain different colors, and how anodizing affects the accuracy of CNC parts.
The Principle of the Aluminum Anodizing Process
The aluminum anodization is an electrolytic cell reaction in principle. The three major components of the electrolytic cell reaction are the external power supply, electrolyte solution, and cathode and anode electrodes. The reaction process of the electrolytic cell is the process by which the current passes through the electrolyte solution and causes the reduction and oxidation reactions at the cathode and anode. The anode of aluminum alloy oxidation is the aluminum alloy to be protected. The cathode is mostly made of 316 stainless steel in the industry, but it can also be made of graphite or lead. According to the types and requirements of anodic oxidation, sulfuric acid, chromic acid, oxalic acid, phosphoric acid, and some organic acids can be used as electrolytes.
The chemical reaction formula of the anode and cathode is as follows:
- Anode: 2Al + 3H2O = Al2O3 + 6H+ + 6e-
- Cathode: 6H+ + 6e- = 3H2
- Overall: 2Al + 3H2O = Al2O3 + 3H2
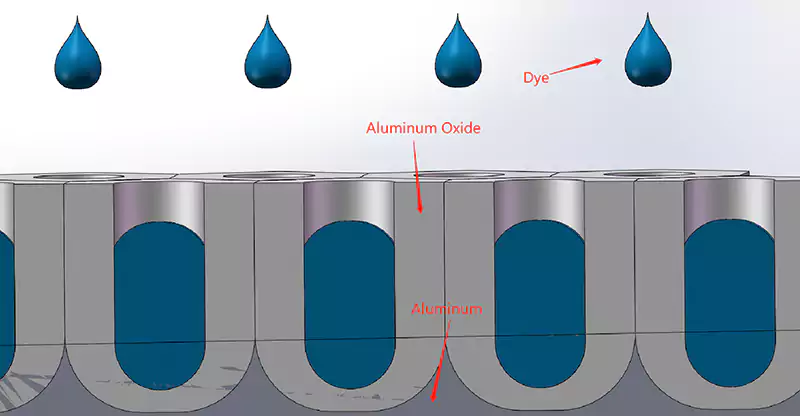
The aluminum oxide film produced by anodic oxidation is a dense porous structure. Dyeing is to infiltrate dyes (metallic salts, organic dyes, etc.) into the porous oxide layer to make the aluminum surface present the required color and luster. The hardness of the aluminum oxide film is much higher than that of the aluminum substrate. The aluminum oxide film insulates the metal substrate from the mediums (air, seawater, etc.) preventing it from oxidation and corrosion of the metal body. It also improves the friction resistance and durability of the metal.

Types of Anodizing Processes
There are many classification methods for aluminum alloy oxidation. The most commonly used at present is to divide it into three categories: type I, II, and III. This classification is mainly based on the specification of Mil-A-8625 (MILITARY SPECIFICATION, ANODIC COATINGS FOR ALUMINUM AND ALUMINUM ALLOYS).
Type I – Chromic Acid Anodizing Process
The conversion oxide film formed by type I anodizing (or chromic acid anodizing) is the thinnest of the three types of anodizing, with a thickness of about. 0002 “-. 0001” (0.5~2.5um). Although the conversion oxide film is thin, it can still provide sufficient corrosion resistance and durability for the aluminum substrate. This oxide film has good elasticity. Due to the large temperature difference in the use environment of aerospace and military products, the product size can change, and thick oxide film has a large chance to crack. Compared with the other two types of oxide film, the chromic acid oxide film is more suitable for such products.
Type I -Colors
Because the chromate oxide film is very thin, the amount of pigment absorbed during dyeing is very small, so the color that can be dyed is very limited. Basically, there are only gray and black options.
Type I Aluminum Anodization vs Aluminum Passivation
Aluminum alloy passivation also uses chromic acid to produce conversion film, but passivation does not need to be electrified, and the conversion film produced can conduct electricity. Type I oxide film is produced by electrochemistry, and the resulting oxide film is not conducive. The oxide film produced by both processes can sometimes be used as a primer for paint and adhesive application.
Type I Anodization Applications
It is also because the chromic acid anodic oxide film is very thin and has little effect on the size of the workpiece, so it is suitable for the surface treatment of precision parts.
Because hexavalent chromium is harmful to the environment, type I aluminum anodization is less and less used in industry. At present, it is mainly used in aerospace, precision parts, or special fields with welding performance requirements.
Type IC – Boric-Sulfuric Acid Anodizing Process
The waste liquid produced by Type I oxidation, which uses chromic acid as the electrolyte, contains hexavalent chromium and is harmful to the environment and toxic to the human body. Borosulfuric acid anodizing is an alternative process to Type I anodizing. The nature of the conversion oxide film produced by this process is very similar to that of chromic acid anodizing. Thin thickness, strong corrosion resistance, elastic, mostly used for aerospace/aircraft parts or precision parts in harsh environments. This process is included in the type IC anodization specification of MIL-A-8625.
Type II – Sulfuric Acid Anodizing Process
Type II anodizing is the most popular and most common type of anodization process. Type II anodizing uses sulfuric acid as the electrolyte solution which produces the porous structure more efficiently. And hence, it is easier to absorb various organic and inorganic dyes. Because type II anodization can produce a good range of different colors, it is not only used to protect aluminum alloy from environmental corrosion and oxidation but also used for decoration. The oxide film produced by the type II anodizing process is thicker than that of type I, about 100-1000” (2.5um~25um). The oxide film produced by type II is harder than that produced by type I, and it is also not conducive.
Type II Aluminum Anodization Applications

Type II anodizing process is suitable for more types of aluminum alloys. It is also cheaper as it does not require the environmental after-treatment required for Type I anodizing. Although the oxide film is relatively thick, the size change of the workpiece caused by the anodizing process can still be controlled to a minimum level through accurate control of parameters such as pickling and oxidation time and voltage, etc. Therefore it is also suitable for the surface treatment of precision parts.
Good corrosion resistance, durability, wide color selection, and low cost make this oxidation process suitable for a very wide range of products. Type II anodization process is used in almost all industries.
Type III – Hard Coat Anodizing Process

Hard coat anodizing is also carried out in a sulfuric acid-based electrolyte like type II anodizing, but in order to obtain a thicker and denser oxide layer, the voltage and current density used in this process are higher, and the concentration and temperature of the electrolyte are lower. The reaction time is much longer.
The thickness of the oxide film produced by type III anodic oxidation can reach thousands of microinches (tens of um), with a hard surface and low porosity.
Type III Anodization Applications
Hard coat anodizing is primarily used on aluminum parts requiring extreme wear resistance, or in corrosive environments where a thicker, harder, more durable coating is required. We have done some aluminum alloy parts for the 5G communication industry, because the industry requires 30 years of weather resistance (30-day salt spray test), we finally adopted type III anodizing as the surface treatment.
Hard coat Anodizing is also used where enhanced electrical insulation is required. Because the oxide layer of hard anodizing has a high thickness, it is occasionally used for repairing parts after parts are worn or machined incorrectly. But this anodizing process is generally not a good option for precision parts.
Type III – Colors

It is very hard to get a totally clear coating for type III anodized aluminum alloy. Depending on the alloy type the oxide coating has different shades even without the dyeing process. 2000 series, green-grayish; 3000, grayish, 5000, dark gray; 6000, dark gray; 7000-series, yellowish green. The only suggested color for hard coating anodizing is black. Dark colors like dark burgundy and dark blue can also be produced but aluminum alloy types and pigments have to be carefully checked and sampled.
Industrial Work Flow of Aluminum anodizing Process

The typical workflow of the type II aluminum anodizing process is as follows.
Racking – Pickling – Etching – Chemical Polishing – Anodizing – Coloring – Sealing – Drying
Racking: Hang parts on racks. The racks will carry electric current to the parts and will be immersed in acid, alkali, and Metallic salt solutions. They are made from titanium alloy.
Pickling: Immerse the parts in an acid or alkaline solution to remove oil, grease, and other impurities so they would not affect the anodizing effect.
Etching: Immerse the parts in the acid solution to remove a very thin layer on the surface, avoid the original oxide layer on the surface from affecting the subsequent anodizing effect, and form a uniform matt surface at the same time. The time of this process will affect the size of the parts, and the etching time of precision aluminum parts needs to be strictly controlled.
Chemical Polishing: This process is mainly used for parts with bright surface requirements. Generally, the mixed solution of three kinds of anhydride is used for chemical polishing. This process can be omitted for parts that require a matt surface.
Anodizing: The workpieces are immersed in sulfuric acid solution, and the pore structure aluminum oxide film is formed on their surface by electrification.
Coloring: Type II oxidation generally adopts dip coloring. The anodized aluminum parts are immersed in a bath containing dye. The dye is adsorbed on the surface of the pore opening of the anodized film. The color produced depends on the type and chemical properties of the dye. Dip coloring is a cheap method that allows manufacturers to paint various colors on aluminum parts. That is why the type II anodizing process is the most popular process for aluminum anodization.
Sealing: After anodic oxidation and dyeing, the aluminum parts are immersed in ionized water or distilled water to seal the porous structure oxide layer. There are three commonly used sealing methods, hot water sealing. Add metal salts such as nickel, magnesium, or cobalt to medium-temperature water to seal. Sealing of room temperature water plus nickel fluoride and other metal salts. The oxide layer after sealing is stronger, smoother, and more durable.
Aluminum Coloring and Lightfastness
There are 4 ways to color anodized aluminum parts. Dip coloring, Electrolytic coloring, Integral Coloring, and Interference Coloring. “Lightfastness is a property of a colorant such as dye or pigment that describes its resistance to fading when exposed to light.”- Wikipedia. A lightfast color or UV-resistant color is a color that does not fade easily, in simple terms.
Dip coloring:
The most common method to color anodized aluminum parts. Anodized aluminum parts are immersed in a bath containing organic pigments to be colored. A wide range of colors can be selected. But not very lightfast. Even black anodized aluminum produced by dip coloring fades to brownish over time.
Electrolytic coloring:
After anodizing, the metal is immersed in a bath containing an inorganic metal salt. Current is applied to embed the metal salt in the base of pores. This is not a typical type II anodization process as current is needed in coloring. Color choices are limited by the metal salts that are suitable for the process, such as Champagnes, Bronzes, Black, Copper, and Burgundies. Inorganic ‘dye’ make the color lightfast, and suitable for outdoor products.
Integral Coloring
In the early 1960s, Alcoa licensed a trademark, Duranodic Integral Color Anodizing process. With the passage of time, there are more and more methods of anodic oxidation dyeing, the Duranodic process became known as “integral coloring.” It simply means anodizing and coloring are integrated into one process.
The electrolyte used in this process is a mixture of sulfuric acid, organic acid, and metallic salts. Since the ‘dye’ used here is inorganic, the color produced is lightfast, but they are limited to Champagnes, Bronzes, Black, and Grays.
It is probably the most costly aluminum anodizing coloring method, as a lot more energy consumption is required. The coat is harder than electrolytic coloring, but it also means more chance of craze.
The process is still used by some architectural aluminum manufacturers, but more and more manufacturers use other alternative methods.
Interference Coloring
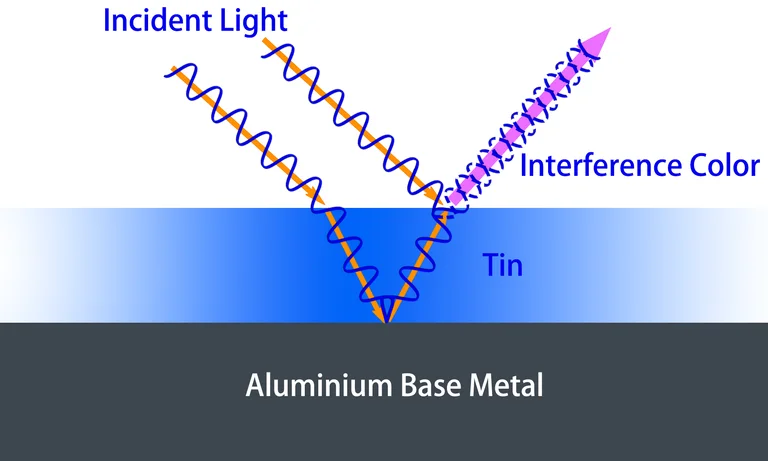
Interference coloring is a method to obtain different colors by using the principle of optical interference. Light has the properties of waves. When two beams of light meet, they overlap each other, some peaks and valleys are strengthened, and some cancel each other. This phenomenon is optical interference.
On the thin oil film floating on the water’s surface, we can see the rainbow color, which is the result of the interference between the light reflected from the water-oil interface and the light reflected from the oil film surface. Because the thickness of the oil film is not controlled, the interference of the two reflected lights is also not controlled, so we see a random rainbow color.
In the anodizing coloring process of aluminum, the desired color can be produced by depositing a metal layer (usually tin) with controllable thickness at the bottom of the porous structure. At this time, the two reflecting surfaces are the aluminum substrate surface and the deposited metal upper surface. The deposited metal layer changes from thin to thick, and the color formed by interference changes from blue, green, and yellow to red. When the thickness exceeds a certain value, the optical interference phenomenon disappears and the color turns to bronze. The interference-colored anodized aluminum parts have an interesting character, when observed from different angles, the color varies. Obviously, this process produces lightfast colors.
This coloring process needs to modify the structure of the porous layer and widen the width of the bottom of the gap to produce an interference effect. In practice, a phosphoric acid anodic oxidation process is added after the sulfuric acid oxidation bath. And the current has to be precisely controlled.
· Aluminum Grade affects Anodized Aluminum Colors
Different aluminum grades affect the anodizing quality differently.
The 2xxx Series Aluminum Alloys:
With high copper content, the anodized layer on this aluminum alloy is softer and has lower corrosion resistance than other alloys, and the anodized aluminum color is easier to rubbered off.
The 3xxx Series Aluminum Alloys:
With high manganese content, the anodized aluminum colors are silver, grayish, or brownish.
The 4xxx Series Aluminum is most used for welding, not for anodizing.
The 5xxx Series Aluminum Alloys:
Alloy 5052 anodizes yellowish color, and 5005 anodizes grayish or brownish color. The color variation is mostly because of their iron and magnesium content.
The 6xxx Series Aluminum Alloys:
This series of aluminum alloys have an excellent anodizing effect, regardless of color and texture.
The 7xxx Series Aluminum Alloys:
The clear anodized 7xxx aluminum appears a brownish or grayish tone. They anodize gray, blue-gray, and brown-black colors.
Summary:
Aluminum anodizing is an important industrial process that improves the physical and aesthetic properties of aluminum products. The process not only improves the aluminum’s durability, corrosion resistance, and appearance but also increases its functionality and versatility. Different anodizing and dyeing processes can be selected according to different needs. Different grades of aluminum alloy affect the anodized aluminum colors differently.
Practice problems:
Capable Machining has years of practice in anodizing aluminum. Here are some problems we have encountered in practice.
- There are no holes in the part. To hang the parts on the racks, it is necessary to have holes in the parts. In the absence of holes, usually, there will be metal wires winding around the parts and then hanging, which will cause a poor-looking surface after anodizing. Or drill “process holes on” the parts, which should be communicated with the designer.
- Rare color and small quantity. One color needs a separate dyeing pool, and there is a certain one-time investment in setting the dyeing pool. If small batches of products are to be dyed with rare colors, the unit cost will be high. Therefore, clear anodizing or black anodizing is generally recommended for small batches.
- Loss of small parts. If the aluminum parts are too small, some of them sometime will be left at the bottom of the pool due to water flow during pickling and cleaning, resulting in a loss. For small batches of parts, abundant quantity should be produced to compensate for the loss. For bulk production, custom-made racks should be ordered to prevent part loss.
FAQs
Q: Can die-casting aluminum be anodized?
A: Die-cast aluminum usually contains high silicon content to ensure good fluidity after melting. During anodic oxidation, aluminum forms a dense oxide film, and silicon is an impurity, which will destroy the consistency of the oxide film. Anodizing of die-casting aluminum is generally not recommended.
By adjusting the concentration of various components in the electrolyte and accurately controlling the current and voltage, die-casting aluminum can also achieve acceptable anodic oxidation results. However, this process needs constant trial error, and analysis, it is not recommended for small-batch products.
Q: Can the anodized layer be removed and re-anodized?
A: Yes, it can be removed by a phosphoric stripping solution but the process ‘eats’ away quite some part size. It is not a good choice if precision is needed.
I have a problem with anodizing Al 1000 series color. I use phosphoric acid and sulfuric acid bath for anodizing and I can not achieve black color anodizing film. I would appreciate if you can help me in this matter.