How Electroplating Works and Where it is Used
Although alchemy may not be real, electroplating is a close alternative for transforming common chemical elements into valuable ones. This involves applying electricity to cover a base metal, such as copper or aluminum, with a thin layer of another metal, such as gold or silver. Electroplating serves many purposes beyond just enhancing the appearance of inexpensive metals. It can also be utilized to make items resistant to rust, and even make plastic resemble metal. How does this fascinating technique function? Let’s delve deeper and find out.
What is Electroplating?
In the electroplating process, an electric current is passed through an electrolyte solution by submerging two electrodes into it and connecting them to a power supply or battery. Both the electrodes and electrolytes are made up of carefully selected elements or compounds. As the electricity travels through the circuit, the electrolyte breaks down, causing some of its metal atoms to deposit onto one of the electrodes, effectively electroplating it. A wide variety of metals can be plated using this method, including copper, gold, silver, zinc, tin, cadmium, nickel, chromium, platinum, and lead.
This process is similar to electrolysis, which involves the use of electricity to break down a chemical solution. Electrochemistry is the study of chemical reactions that are caused or sped up by electricity to make products that are useful in science or industry. This includes electroplating, electrolysis, and how batteries work.
How Does Electroplating Work?
Here are the basic steps involved in electroplating:
Electrolysis
The electroplating process is based on the principle of electrolysis, which is a chemical reaction or the decomposition of a substance by an electric current. When a direct current (DC) is passed through a solution containing ions of the metal to be plated, the metal ions become reduced (i.e. gain electrons) at the cathode (negative electrode). They are deposited on the surface of the cathode (the parts to be plated) being plated. Meanwhile, the anode (positive electrode), which is normally the plating metal, loses electrons and turns into dissolved metal ions.
Electrodes
Electrodes are the conductive materials used to carry the electric current into the electrolyte solution. The cathode is typically made of the metal to be plated, while the anode is usually made of the plating metal.
Plating bath
The plating bath is the solution containing the metal ions to be plated onto the object. It typically consists of a salt of the metal in question dissolved in water, along with various additives to improve the plating process, such as buffers to control the pH of the solution, surfactants to reduce surface tension, and complexing agents to help keep the ions in the solution.
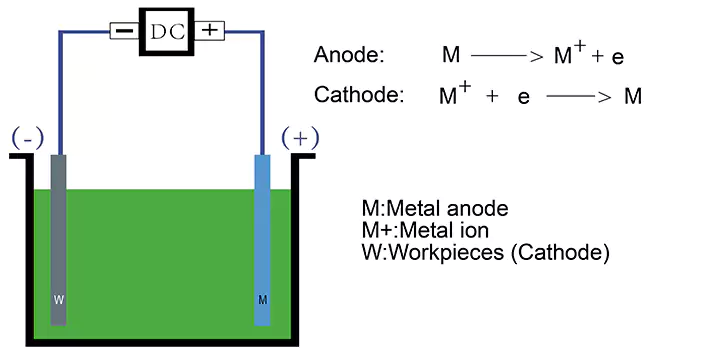
Deposition of the metal
As the electric current flows through the electrolyte solution, metal ions are attracted to the cathode and deposited onto the surface of the object being plated. The rate of deposition depends on a number of factors, such as the current density (the amount of current per unit area), the concentration of metal ions in the plating bath, and the temperature of the solution.
Types of Electroplating
Decorative electroplating is primarily used to improve the appearance of objects. It involves depositing a thin layer of more precious metal, such as gold or silver, onto a less expensive metal, such as copper or nickel, to give it a decorative finish. Decorative electroplating is commonly used in the jewelry industry, as well as for home decors, such as doorknobs, light fixtures, and picture frames.
Functional electroplating, on the other hand, is used to improve the properties and performance of a material. It involves depositing a layer of a specific metal onto an object to enhance its corrosion resistance, wear resistance, conductivity, or other properties. Functional electroplating is used extensively in the automotive industry to improve the durability of engine parts and prevent corrosion. It is also used in the aerospace industry to improve the performance of components, such as turbine blades and hydraulic parts, and in the electronics industry to improve conductivity and prevent corrosion.
Metals for Electroplating
A wide range of different metals can be used for electroplating, including:
Gold
Silver
Copper
Nickel
Chromium
Zinc
Cadmium
Tin
Lead
Rhodium
Palladium
Platinum
Iron
Cobalt
Aluminum
The choice of metal depends on the specific properties required for the application. For example, gold and silver are often used for decorative purposes due to their lustrous appearance, while copper plating and nickel plating are commonly used in the electronics industry due to their conductivity. Chromium and zinc are frequently used to prevent corrosion, and tin and lead are used for soldering. Copper plating is sometimes used as an undercoat to increase the plating adhesion of other metals’ plating such as nickel. The specific metal used for electroplating also affects the cost and environmental impact of the process.
Electroplating and Tolerances
Designers need to understand that the tolerance of parts that have been electroplated can be affected by several variables, including the type of metal used for plating and the plating process itself. Different chemistries and processes will produce different results when it comes to coating thickness uniformity (CTU) as well as overall part tolerances. Below are a few things that could affect the part tolerance by the electroplating process.
Edge build-up
Edge build-up is common in electroplating, it occurs when the edges of a part are thicker than the middle as the edges share more plating current distribution. This can cause a poor fit between components. From a design point of view, use rounded edges as much as possible.
Barrel Plating vs Rack Plating
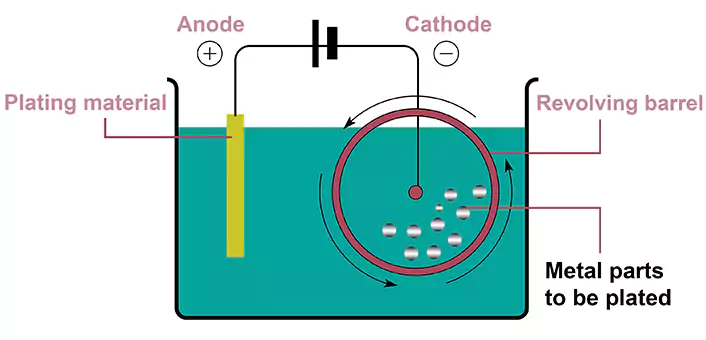
In barrel plating, parts are placed into a rotating barrel filled with the plating solution, which ensures uniform coverage of the substrate. The rotation of the barrel is also important for achieving good CTU (Coating Thickness Uniformity). Additionally, since part size and shape don’t matter in this process, they can be used to plate small and intricate components as well as large and complex ones. The limitation of the part size of a barrel plating depends only on the barrel size. Because CTU is pretty good, it is easier to control tolerances when applying barrel plating.
In rack plating, parts are hung on racks and immersed in the plating solution. The near end (close to the power supply) is normally plated thicker while the far end is plated thinner. The bad CTU in rack plating can cause problems with tolerance control. The designer has to give more tolerance when a rack plating is in choice, or the grinding process after rack plating has to be considered to keep a tight tolerance.
Hard-to-flow areas
If the shape of the part is complex, some corners form semi-closed areas. During the electroplating process, the metal ions in the electroplating solution in these hard-to-flow areas cannot be replenished in time, and a very thin electroplating layer will be formed, which will affect the precision and appearance of the parts.
It is important to factor these considerations into the design process in order to ensure proper performance from electroplated components.
Electroplate Plastics
Plastics have become one of the most versatile and widely-used materials due to their affordability, flexibility, and lightweight properties. However, to some, the low cost and common appearance of plastic may be seen as a disadvantage. To address this issue, a solution is to electroplate a thin film of metal onto plastic surfaces, giving them an attractive and shiny appearance while retaining the practical benefits of plastic. This process can be applied to various types of plastics, such as ABS, nylon, polycarbonate, phenolic plastics, and urea-formaldehyde. Plated plastic parts are commonly found in plumbing, household, automotive, and electrical components, providing a rustproof and cost-effective alternative to all-metal parts that require no additional polishing after plating.
How are Plastics Electroplated?
The challenge with electroplating plastics is their lack of electrical conductivity. However, this does not render the process impossible, and additional steps are taken to make the plastic surface electrically conductive. The first step involves thoroughly cleaning the plastic to eliminate any surface impurities like grease, dirt, or marks.
The plastic is then treated with acid and a catalyst to prepare the surface for the metal coating. Next, the plastic is immersed in a bath containing copper or nickel (copper being more common), creating an ultra-thin metal layer that is less than one micron thick. After this initial treatment, the plastic is ready for electroplating and can be coated with a thickness ranging from 10-30 microns depending on the application’s wear and tear requirements.
Advantages of the Electroplating process
The advantages of electroplating are the following:
Improved corrosion resistance
Nickel and chrome can generate a dense oxide layer in the air, and nickel or chrome electroplating can improve the corrosion resistance of metals, protecting them from damage caused by exposure to moisture and other environmental factors. Gold is an inert metal, it does not oxide easily, gold plating isolates the base metal from external media and hence improves the corrosion resistance too. For example, soil moisture sensors need to resist corrosion from the soil and keep the surface’s electronic conductivity, gold plating is the only solution.

Improved wear resistance
Electroplating can also improve the wear resistance of metals, making them more durable and longer-lasting. A metal electroplating surface is certainly harder to scratch than a paint or powder-coating surface.
Improved appearance
Electroplating can enhance the appearance of metals, giving them a shiny and attractive finish.
Reduced friction
Electroplating can reduce friction between moving metal parts, making them smoother and more efficient.
Versatility
Electroplating can be used on a wide range of materials, including metals, plastics, and ceramics, making it a versatile coating option for many industries.
Cost-effective
Electroplating is a relatively cost-effective method of coating metals compared to other processes like painting or powder coating.
Disadvantages of Electroplating
However, it also has several disadvantages:
Environmental concerns
The electroplating process involves the use of chemicals, which can be harmful to the environment if not handled properly. Waste disposal and chemical spills can lead to pollution and contamination of soil, water, and air.
Limited material options
Not all materials can be electroplated, and the process may not be suitable for certain materials or shapes. Some materials may require pre-treatment or special processes to prepare them for electroplating.
Health and safety risks

The chemicals used in electroplating can be hazardous if not handled properly, and workers may be at risk of exposure to fumes, dust, and other hazards.
Energy consumption
Electroplating can consume a significant amount of energy, contributing to greenhouse gas emissions and other environmental impacts.
Conclusion
In summary, electroplating is a process that involves passing an electric current through an electrolyte solution to coat one metal with a thin layer of another metal. This process has a range of applications in various industries, including decorative and functional coatings, improved corrosion and wear resistance, and reduced friction.
The electroplating process begins by cleaning the substrate and then treating it with an acid etch and catalyst to make it electrically conductive. The substrate is then dipped in a bath of the desired metal, which is deposited onto the surface through the application of an electric current.
Electroplating is widely used in industries such as automotive, aerospace, electronics, and jewelry. It is essential to produce parts that are corrosion-resistant, wear-resistant, and attractive. The process is also used in creating alloys and in making plastic and other non-metallic materials look like metal.
Overall, electroplating plays a critical role in various industries, contributing to the production of durable and aesthetically pleasing products. The process has its advantages and disadvantages, including environmental concerns, cost, limited material options, and health and safety risks, which need to be managed carefully to ensure safe and sustainable practices.