Passivation: Shielding Metals from Rust and Decay
Introduction
Passivation is a commonly used method for treating metal surfaces. Like many other surface treatment processes, its primary purpose is to protect metal surfaces from rust and corrosion. What sets the passivation process apart from many other surface treatment methods is that its protective layer is entirely formed through chemical reactions involving the base metal itself, without the need for additional metal plating or paint coatings.
In this article, we’ll delve into the world of passivation, exploring what it is, how it works, and why it’s essential for metals like aluminum and stainless steel. So, let’s embark on this journey to understand how passivation maintains the strength and longevity of our reliable metals.
What is passivation?
Passivation is essentially a chemical process. It uses chemical reactions to create a thin, tight oxide layer on a metal’s surface. This oxide layer might be exceedingly thin, but it’s a strong shield against environmental factors like oxygen and other substances that could cause rust or oxidation by interacting with impurities on the metal’s surface. The ultimate goal of passivation is to shield and preserve the base metal. It’s important to note that the precise chemical reactions and chemicals used for the passivation process can vary depending on the specific metal involved.
In the forthcoming sections, we will explore the specific applications of passivation, from its role in preserving aluminum to its effectiveness in safeguarding stainless steel.
Aluminum Passivation
When exposed to the air, pure aluminum, such as 1000-series aluminum alloys, forms a sufficiently dense oxide layer on its surface. This layer acts as a natural shield, halting further oxidation. What’s intriguing is that this oxide layer possesses a self-repairing capability, growing back even after scratches or damage.
This corrosion-resistant attribute isn’t limited to pure aluminum alone; it extends to various other aluminum alloys, including the 2000, 3000, 5000, 6000, and 7000 series. They all have the property of producing a protective oxide film on their own to some extent.
However, in the real world, components often face environments far from pure air—environments filled with humidity, salt, water, oil, and various chemicals, all of which can intensify the oxidation and corrosion of aluminum alloys.
To offer enhanced protection for aluminum alloys, industrial practices come into play. Common surface treatment methods include Alodine, anodizing, painting, powder coating, electroplating, and more. Broadly speaking, both Alodine and anodizing fall within the realm of passivation, as they don’t necessitate the application of additional coating materials. Instead, these surface treatments rely on the chemical reactions inherent to the aluminum alloy itself to create a passive layer.
However, because anodizing is so well known and the anodizing process involves the use of electricity, when we talk about the passivation of aluminum alloys within an industrial context, we’re often referring to Alodine.

Alodine: the Aluminum Passivation process
Alodine is also called “chem film” or “chromate conversion coating”. The principle of the Alodine process is to create a passive layer on the surface of aluminum. This passive layer primarily consists of a compound called aluminum chromate (Al2Cr3O12). The formation of this layer occurs through a chemical reaction between the aluminum surface and the chemicals present in the Alodine solution, which typically contains hexavalent chromium compounds (for example, Na2Cr2O7), and acids. The hexavalent chromium protective film appears light yellow and transparent. This process was used for many years before more environmentally safe methods emerged.
Typical reaction:
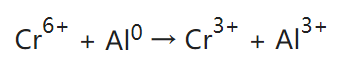
Since hexavalent chromium is carcinogenic and not environmentally friendly, most Alodine nowadays uses trivalent chromium. The active ingredients of trivalent chromium Alodine solution mainly include trivalent chromium compounds like chromium chloride, chromium sulfate, chromium nitrate, chromium phosphate, Chromium acetate or potassium chromium sulfate, as well as oxidants, complexing agents, etc. The protective film of trivalent chromium conversion is consistent with chromium(III) oxide Cr2O3, or mixed (III)/(VI) oxide, with very little Al2O3. It appears transparent and colorless, or light blue.
Typical reaction:
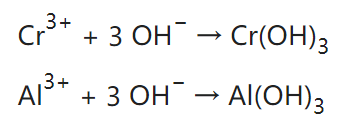
The key principle behind Alodine lies in the ability of hexavalent or trivalent chromium to interact with the aluminum surface and promote the development of a tightly adhering, self-healing oxide layer. This oxide layer acts as a barrier, preventing the metal from further oxidation and corrosion. Alodine produces very thin protective films, with typical thicknesses around 200–300 nm.
Characteristics of Alodine Conversion Coating:
Corrosion Resistance and Anti-Rust Properties: Alodine is well-known for its excellent corrosion resistance and anti-rust performance. It is frequently used on aluminum alloy components in communication, military, and aerospace applications. Its corrosion resistance is remarkable, with the ability to withstand salt spray tests for up to 72 hours.
Electrical Conductivity: Alodine stands out as one of the few surface treatment methods that preserves the electrical conductivity of the metal. It is commonly employed for surface treatments of electronic devices that require grounding, such as the casings of electronic equipment or connectors used in electronic components.
Pre-Treatment for Other Surface Treatments: In the initial stages of forming the Alodine conversion coating, it exhibits a high-viscosity gel-like state. This state is conducive to the adhesion of coating materials used in other surface treatments, making Alodine a valuable pre-treatment option before processes like painting or anodizing.
Minimal Impact on Precision: The aluminum passivation process generates an extremely thin passive layer, which has a negligible impact on the dimensions of components. As a result, it is commonly employed for the passivation of precision components where maintaining exact dimensions is critical.
Stainless Steel Passivation
Stainless Steel’s Corrosion Resistance
The reason why stainless steel is stainless’ is mainly due to the chromium content in the stainless steel alloy. Chromium can form a chromium oxide layer (Cr2O3) in the air to prevent further oxidation of the base material. The nickel content of 300 and 600 series stainless steel has a similar effect. But in some cases, stainless steel will still be rusted. One of the more common rusts is due to trace amounts of iron or impurities embedded in the stainless steel surface grain boundaries, and water molecules corrode this part of the iron. The stainless steel surface will therefore show spots of rust. This phenomenon is called “rouging“. “Rouging” often occurs on stainless steel surfaces after welding, machining, or heating. Environmental factors such as detergents, bleach, or seawater can also exacerbate the corrosion of stainless steel. Therefore, the passivation process is still a very popular option for stainless steel products for household or industrial use.

Stainless Steel passivation principle
As mentioned earlier, the main reason stainless steel can rust is the presence of “free iron” or other impurities on its surface. So, the simplest and most direct way to boost rust resistance is to get rid of these ‘free iron’ impurities from the stainless steel surface, thereby increasing the chromium content on the surface.
According to the ASTM A967 standard, passivation is defined as:
“The chemical treatment of stainless steel with a mild oxidant, such as a nitric acid solution, for the purpose of the removal of free iron or other foreign matter.”
In the ASTM A380 statement, passivation is defined as:
“The removal of exogenous iron or iron compounds from the surface of stainless steel by means of a chemical dissolution, most typically by treatment with an acid solution that will remove surface contamination but will not significantly affect the stainless steel itself … for the purpose of enhancing the spontaneous formation of the protective passive film.”
In simpler terms, stainless steel passivation uses an acid solution to remove ‘free iron‘ and other impurities from the stainless steel surface while keeping the chromium content intact. During the industrial passivation process, stainless steel is cleaned several times, including degreasing, pickling, and rinsing. It is then passivated using nitric acid or citric acid solutions. Nitric acid passivation has been used in the industry for a long time, but it poses environmental pollution and safety concerns. Since the 1990s, more and more manufacturers have been switching to citric acid passivation.
After passivation, stainless steel forms a protective film in about 24 to 48 hours. Thanks to its relatively higher chromium content on the surface, passivate stainless steel parts develop a thicker and denser chromium oxide layer, effectively preventing the occurrence of ‘rouging.’

Characteristics of Stainless steel passivation
Electrical Conductivity
Passivated stainless steel maintains its electrical conductivity. This happens as it forms a thin oxidized layer through a chemical reaction, ensuring a steady and high level of conductivity.
Passivation: Not an Electrolytic Process
It’s essential to note that stainless steel passivation is a purely chemical reaction, not an electrochemical one. Unlike processes such as anodizing or electroplating, chemical passivation doesn’t require electrical power throughout its entirety. It’s a more energy-efficient surface treatment method.
Passivation Preserves the Original Color
Passivation leaves the stainless steel with a transparent and colorless oxide layer. It’s remarkably thin and doesn’t alter the stainless steel’s appearance, in contrast to methods like painting or powder coating that change the surface’s color.
Where is stainless steel passivation used?
Stainless steel passivation finds application in various industries and settings, including:
Aerospace: Critical stainless steel parts in aircraft and spacecraft benefit from passivation to withstand the demanding conditions of flight.
Medical Devices: Surgical instruments and medical equipment rely on passivation for corrosion resistance and biocompatibility.
Food and Beverage: Stainless steel equipment used in the food processing and brewing industries utilizes passivation to maintain hygiene and prevent contamination.
Pharmaceuticals: Equipment in pharmaceutical manufacturing requires passivation to meet stringent cleanliness and corrosion resistance standards.
Automotive: Passivation is used in manufacturing and assembling automotive stainless steel parts, ensuring their durability and longevity.
Chemical Processing: Stainless steel vessels and equipment in chemical plants rely on passivation for corrosion resistance.
Marine Industry: Passivation helps corrosion-resistant marine equipment and structures exposed to saltwater.
Construction: Stainless steel used in architectural elements, such as handrails and building facades, benefits from passivation to maintain aesthetics and structural integrity.
Electronics: Passivation is crucial for components in electronics, offering protection against environmental factors.
Oil and Gas: Passivate stainless steel pipelines and equipment in the oil and gas industry are used to resist corrosive substances and harsh environments.
In essence, stainless steel passivation is essential wherever corrosion resistance and longevity of stainless steel components are required, spanning diverse sectors and applications.
In Conclusion: Protecting Metals
In the world of metal passivation, we’ve uncovered the processes that guard metals against corrosion. Whether it’s aluminum or stainless steel, these techniques enhance their resistance, ensuring they last in various applications. Passivation is essential for preserving metal integrity, be it in household items or aerospace components.
For precision machining services for your metal components, visit CapableMachining.com. Our commitment to precision aligns with passivation’s principles, ensuring your components excel. Discover how our expertise can elevate your projects today.